Pharmaceutical Quality Assurance - Unit 1
Syllabus
Quality Assurance and Quality Management concepts: Definition and concept of Qualitycontrol, Quality assurance and GMP
Total Quality Management (TQM): Definition, elements, philosophies
ICH Guidelines: purpose, participants, process of harmonization, Brief overview of QSEM, with special emphasis on Q-series guidelines, ICH stability testing guidelines
Quality by design (QbD): Definition, overview, elements of QbD program, tools
ISO 9000 & ISO14000: Overview, Benefits, Elements, steps for registration
NABL accreditation: Principles and procedures
PDF PREVIEW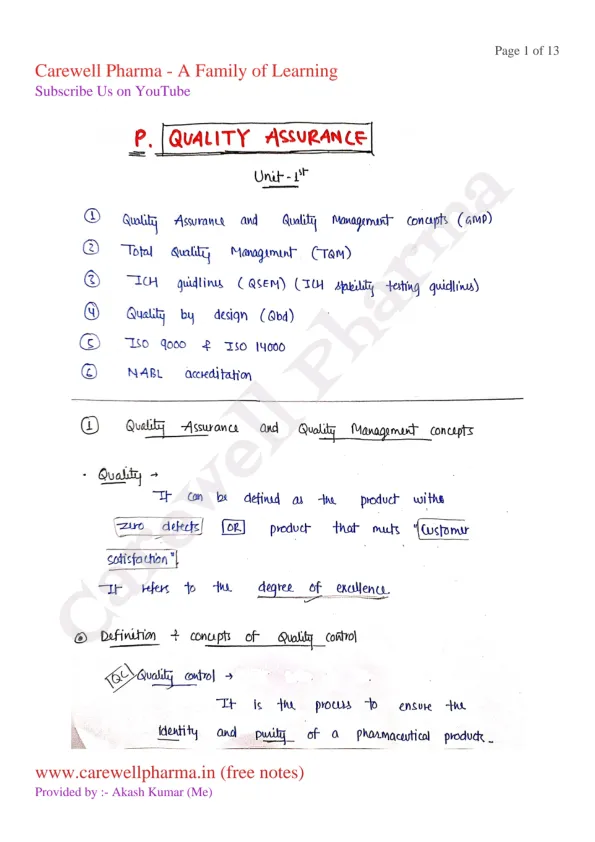
Download PDF